Open-angle glaucoma: Causes, symptoms and treatment
What is primary open-angle glaucoma (POAG)?
Primary open-angle glaucoma (POAG) is the most common type of glaucoma. It usually occurs when pressure within the eye has slowly gotten higher and higher over time. If eye pressure rises too much, it can damage the optic nerve and lead to permanent vision loss.
When glaucoma is called “primary,” it means it doesn’t have an identifiable cause. It’s called “secondary” when it’s caused by a known condition or issue. Primary and secondary types can both be open-angle or closed-angle.
There is no cure for glaucoma, which means it requires lifelong treatment. However, POAG usually responds well to treatment when detected early. Having regular comprehensive eye exams can lead to early detection, which can help prevent vision loss.
Open-angle glaucoma vs. closed-angle glaucoma
Open-angle and closed-angle are the two basic categories of glaucoma. While they may be confused with one another, they have some key differences.
The first key difference is what leads to the increase in eye pressure.
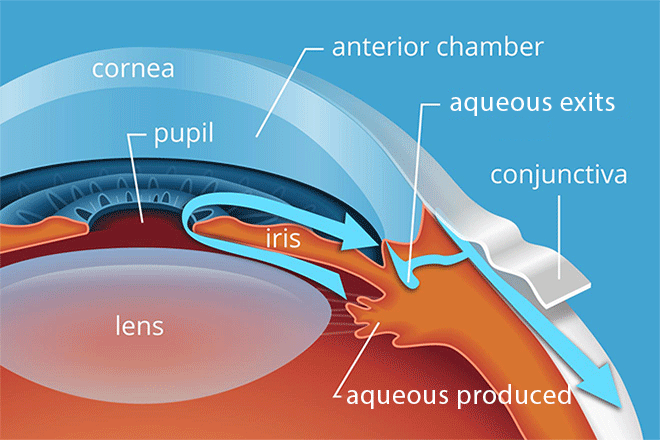
The ciliary body is a structure that sits directly behind the iris (the colored part of the eye). One of its jobs is to create an important fluid called aqueous humor. Aqueous humor flows through a specific route into the front of the eye (the anterior chamber). This route allows aqueous humor to send important nutrients and oxygen to other parts of the eye, such as the lens and cornea.
Aqueous humor also regulates intraocular pressure (IOP) — the medical name for eye pressure.
Once the aqueous humor has run its course, 80%-90% of it drains through a spongy tissue called the trabecular meshwork. This meshwork is located at the bottom of the eye’s drainage angle, where the cornea meets the iris. In a healthy eye, this is a constant process. The ciliary body is always producing aqueous humor, and aqueous is always draining through the trabecular meshwork.
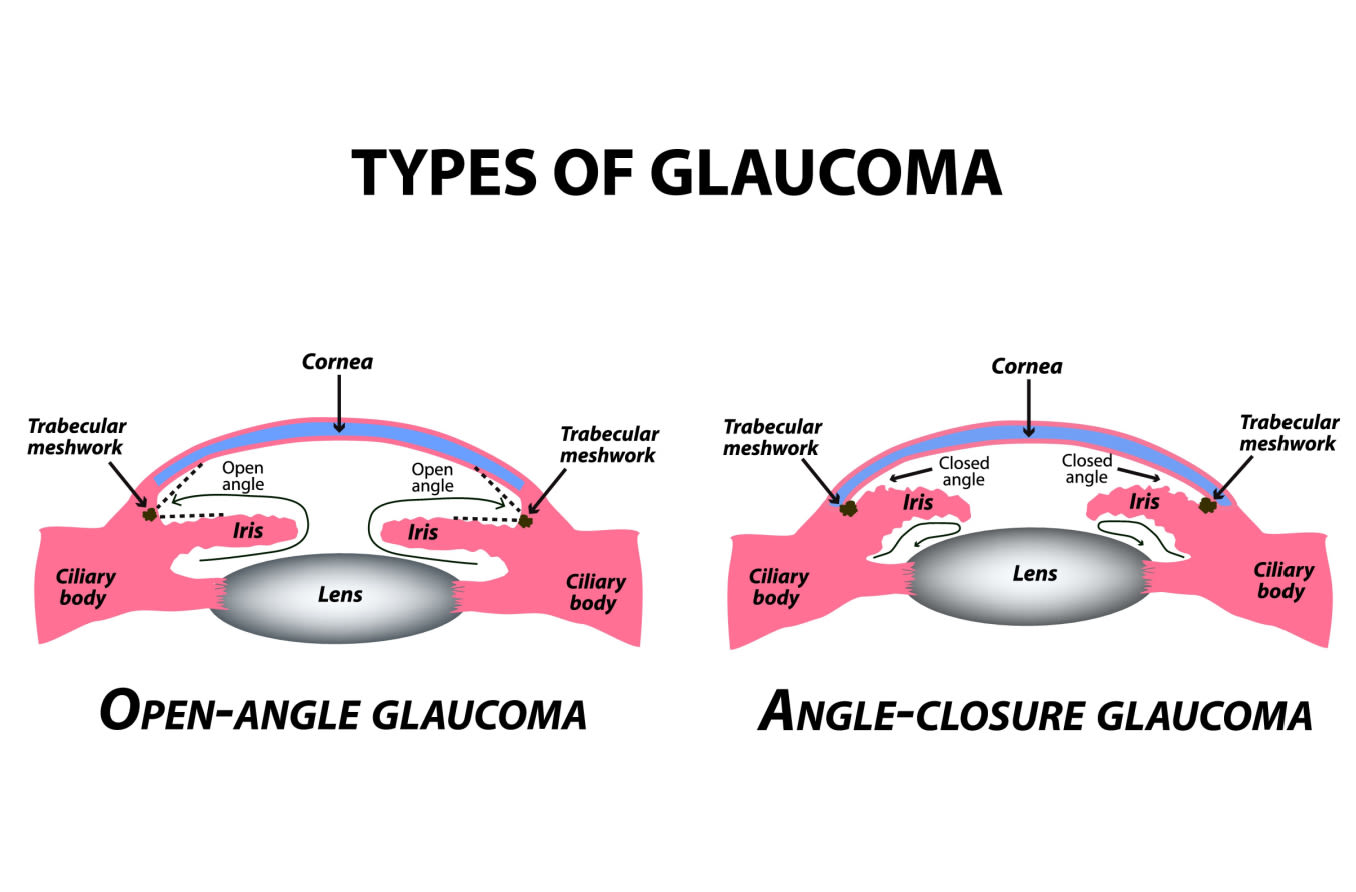
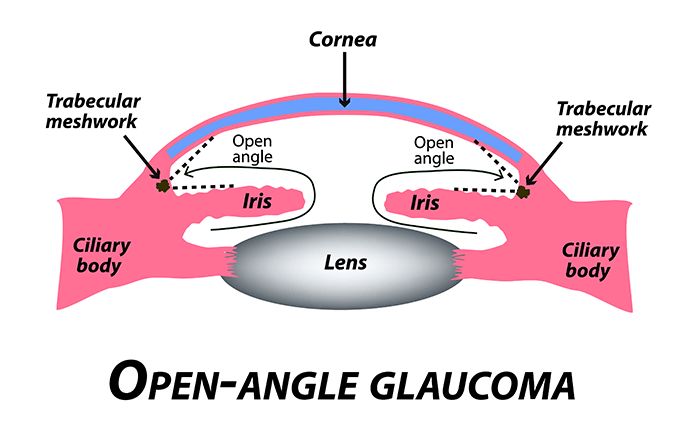
In primary open-angle glaucoma, the drainage angle appears normal and clear. But there is often a blockage deeper down in the channel — think of a clogged pipe that prevents your sink from draining.
Experts believe a problem develops in the trabecular meshwork that slows down how much aqueous can pass through it. Over time, IOP rises because the aqueous outflow can’t keep up with the production.
In angle-closure glaucoma, things can happen much more quickly. This type usually develops when pressure behind the iris pushes the iris forward. The iris then blocks some or all of the drainage angle.
If the angle narrows, it limits the amount of fluid that makes it to the trabecular meshwork. When the angle closes, it blocks any aqueous from draining. In these circumstances, aqueous is still being produced in full force, yet none of it is able to drain. This is why IOP can spike very rapidly.
LEARN MORE about narrow- and closed-angle glaucoma
Another key difference is in their rates of progression.
Open-angle glaucoma is considered chronic because IOP generally rises slowly.
Angle-closure glaucoma may be chronic or acute. Chronic angle-closure (sometimes called narrow-angle or “creeping” glaucoma) is usually gradual, like POAG. Acute angle-closure is sudden and causes a significant increase in eye pressure.
While both types need to be treated by an eye doctor, acute angle-closure glaucoma is a medical emergency. Not seeking immediate medical attention for angle-closure can result in permanent vision loss.
There are also important differences in their symptoms.
Primary open-angle glaucoma has no early symptoms. Vision loss is often the first indication that something is wrong. But by the time vision loss is noticeable, the glaucoma is already in an advanced stage. Extensive and irreversible damage has already occurred.
Symptoms of angle-closure glaucoma can happen much earlier in the disease. They may include eye pain, nausea, severely blurred vision, headaches, and halos or “rainbows” around lights in dim conditions.
Causes of primary open-angle glaucoma
There is no known cause for primary open-angle glaucoma. This is why it’s called “primary” — it’s not caused by (or secondary to) anything else.
Most cases of POAG develop when the drainage channels in the eye don’t let enough fluid through. Experts do understand that the problems occur on a microscopic level, but they are still unsure what leads to them in the first place.
While there’s no clear, known cause for primary open-angle glaucoma, there are several known risk factors. The most important risk factor is having high IOP. Others include family history, cornea thickness, age and certain refractive errors, such as high myopia.
LEARN MORE about how and why glaucoma may develop
Signs and symptoms
An unfortunate aspect of open-angle glaucoma is that it has no early symptoms. This is true for both primary and secondary forms of the disease. Patients don’t usually realize something is wrong until they notice vision loss.
Vision loss from glaucoma usually begins in the outer edges of your visual field and slowly creeps into central vision. Because it begins in your peripheral vision, it is nearly imperceptible for a long time.
As the disease advances, more of your visual field will be lost. It may start to feel like you’re looking through a tunnel. In late stages, glaucoma affects central vision, eventually leading to complete blindness.
Once open-angle glaucoma is detected, treatment can help prevent further vision loss. However, it cannot repair the damage that’s already happened. This is why regular eye exams are so important — an eye exam can detect early signs of glaucoma. Early detection can allow you to start treatment before vision loss occurs.
Diagnosis and treatment
An eye doctor can diagnose primary open-angle glaucoma using a series of tests. They may suggest running the tests if you have risk factors for POAG, even if you don’t have symptoms. Some of the major risk factors include:
Having high eye pressure (IOP)
Being aged 60 or older
Having Black or Latin American ancestry
There are several common tests an eye doctor may use to diagnose or monitor POAG:
Dilated eye exam – This is also called a fundus exam or ophthalmoscopy. Pupil dilation allows the doctor to get a much better look at the retina and optic nerve. The exam is painless, but pupil dilation will make you sensitive to light and can blur your vision for a few hours.
The eye doctor will use a magnifying tool with a light to assess your optic nerve, including its shape and color. If they believe the optic nerve looks unusual, they may have you undergo other testing. These tests may include tonometry, perimetry, gonioscopy, pachymetry or optical coherence tomography (OCT).
Tonometry – Tonometry testing measures intraocular pressure (IOP). This lets the doctor assess what risk there is for POAG or how well glaucoma treatment is working.
Before the test, the eye doctor or technician applies numbing drops to the patient’s eyes.
The patient usually sits in an exam chair, resting their chin and forehead against a slit lamp. A slit lamp is a microscope with a bright light that is often used during eye exams.
The eye doctor then uses a device called a tonometer to put a small amount of pressure on the eye. The tonometer measures intraocular pressure by gauging how much resistance it feels.
Sometimes, this test is done with an air puff. The air puff test isn’t as accurate as other methods, but it is a “non-contact” test and doesn’t require numbing drops. This can make it a good option for screening with some patients.
Normal eye pressure is between 12 mmHg and 21 mmHg. However, someone with an eye pressure within this range can still develop glaucoma. This is called normal-tension glaucoma.
Perimetry – Also known as visual field testing, this test creates a “map” of the patient’s visual field. Results can determine whether glaucoma has affected a patient’s vision. It can also measure the extent of a patient’s glaucoma-related vision loss.
The test requires the patient to look straight ahead at a screen. As they look forward, little lights pop up in different areas of their peripheral vision. The eye doctor can map out the patient’s visual field based on when and where the patient can see the lights.
Gonioscopy – This test lets the eye doctor measure how open the drainage angle is. They may do this test if a patient has other signs of glaucoma or if they have an eye infection or injury.
To check the drainage angle, the eye doctor numbs the eye with numbing eye drops. Then they will place a tool called a gonioscope on the eye.
A gonioscope rests on the eye sort of like a contact lens. However, it’s raised far enough off the eye that the doctor can hold onto and direct it where they need to focus. The gonioscope is mirrored, which allows the eye doctor to look at the drainage angle and see how open it is. If the angle is narrow or closed, it tips the doctor off to angle-closure glaucoma. If the angle is wide open, it may indicate POAG.
Pachymetry – Pachymetry measures how thick the patient’s cornea is. The cornea is the clear dome in front of the iris and pupil.
Before this test is done, the patient will have numbing eye drops applied to ensure comfort. The eye doctor will then place a little probe called a pachymeter on the front of the eye. The probe uses ultrasound to measure corneal thickness.
Measuring corneal thickness can help determine a patient’s glaucoma risk. Having a cornea that is thinner than average is a risk factor for developing the disease.
Optical coherence tomography (OCT) – OCT is a type of imaging that maps out the contour and thickness of the optic nerve tissue. It can be an effective way to detect very slight thinning or damage in this tissue in the early stages of glaucoma. Comparing OCT images taken over time is also very helpful in monitoring for progression of the disease.
OCT is painless and non-invasive and takes about 10 minutes. The patient sits facing the imaging machine with their head resting on chin and forehead supports. As the patient focuses on a target, the machine scans each eye one at a time to create the images.
If one or more of these tests confirm a POAG diagnosis, your eye doctor will likely recommend prescription eye drops and/or laser surgery.
Most cases of open-angle glaucoma can be managed with medicated eye drops. Laser surgery can also be effective for POAG and is becoming a common first-line treatment. However, some cases may require oral medication or conventional surgery.
Eye drops
Eye drops are often an eye doctor’s first recommendation to manage primary open-angle glaucoma. Most drops only need to be applied once or twice per day, which makes them a convenient treatment option.
There are many types of glaucoma eye drops, but they all work in different ways to lower IOP. Some eye drops work by helping the eye drain aqueous humor more effectively. Examples of this include:
Miotic or cholinergic agents, such as pilocarpine (brand name: Isopto Carpine)
Prostaglandins, such as travoprost (Travatan Z), latanoprost (Xalatan), bimatoprost (Lumigan) and tafluprost (Zioptan).
Nitric oxides, such as latanoprostene bunod (Vyzulta)
Rho kinase inhibitors, such as netarsudil ophthalmic solution 0.02% (Rhopressa)
Your doctor may also prescribe eye drops that help limit the amount of aqueous humor your eye produces. Eye drops that achieve this include:
Beta blockers, such as timolol (Timoptic or Istalol) and betaxolol (Betoptic)
Alpha-adrenergic agonists, such as brimonidine (Qoliana or Alphagan P) and apraclonidine (Iopidine)
Carbonic anhydrase inhibitors, such as brinzolamide (Azopt) and dorzolamide (Trusopt)
It’s common for patients to need more than one type of eye drop to manage open-angle glaucoma. In this case, their eye doctor may prescribe a combination eye drop. These types of drops provide the benefits of two or more medications in a single solution. Commonly prescribed combination eye drops include:
Brimonidine and brinzolamide (Simbrinza)
Timolol and dorzolamide (Cosopt)
Timolol and brimonidine (Combigan)
Netarsudil and latanoprost (Rocklatan)
Oral medications
Though rare, it’s possible for your eye doctor to prescribe oral medication to treat glaucoma. This is often reserved for patients who don’t like using eye drops or who can’t use them consistently. Oral medication can also be an option when eye drops alone are not effectively lowering IOP.
Two common oral medications for glaucoma are acetazolamide and methazolamide. Both of these medications are carbonic anhydrase inhibitors. Carbonic anhydrase is a protein that aids in fluid secretion throughout the body, including the eyes.
By limiting this protein, they reduce the amount of aqueous fluid produced by the eye. In turn, this can help lower IOP.
LEARN MORE about glaucoma medications
Surgeries
Glaucoma surgery is an option when eye drops and oral medication don’t work to lower IOP enough. Glaucoma surgery usually has positive results, but most patients do still need eye drops and/or oral medication afterward.
Laser surgery
Different types of laser surgeries can treat different types of glaucoma. They are often used to treat open-angle glaucoma, especially in early or less severe cases. The most common laser procedures for primary open-angle glaucoma include:
Selective laser trabeculoplasty (SLT)
Argon laser trabeculoplasty (ALT)
Laser cyclophotocoagulation (CPC)
During an SLT, the surgeon uses a laser to create new drainage channels in the trabecular meshwork. SLT is the most common laser treatment for POAG. It was recently shown to be more effective and more convenient than eye drops as a first treatment option.
ALT is similar to SLT except the lasers are higher energy and less precise. Like SLT, it opens up new channels in the trabecular meshwork to promote better drainage. Intraocular pressure is successfully lowered in around 75% of patients who’ve had an ALT.
In CPC, the lasers target the ciliary body to inhibit its ability to make aqueous fluid. Doctors usually reserve CPC for later-stage treatment since it involves damaging parts of the ciliary body. Additionally, these damaged areas of the ciliary body may regenerate. This means that CPC often has to be redone.
Minimally invasive glaucoma surgery (MIGS)
MIGS procedures are more invasive than laser treatment but much less so than conventional surgery. They are options for certain patients who haven't had ideal results with medication or laser surgery.
MIGS are appealing because they can lower IOP with very little recovery time or follow up. However, many types of MIGS are only done in combination with cataract surgery.
Two common MIGS for open-angle glaucoma are the trabectome and the iStent trabecular bypass.
Trabectome surgery involves removing a small amount of tissue from the trabecular meshwork. The surgeon uses a tiny tool called a trabectome, which removes the tissue and also irrigates and cauterizes the area.
The incision is made in the cornea and is much smaller than what’s made during conventional surgery. This means there are often fewer risks for the patient. Trabectome can be done on its own, without cataract surgery.
The iStent procedure involves placing a nearly microscopic tube in the eye's drainage structures. The surgeon makes a small incision in the cornea and then uses a special device to inject the iStent into place.
The tube allows aqueous fluid to bypass the eye’s natural drain, which helps lower IOP. This procedure can only be done during cataract surgery.
Conventional surgery
Conventional glaucoma surgery is more complex and invasive than laser or MIGS procedures. Since it’s more invasive, it usually takes longer to perform and has a longer recovery period. The two most common conventional procedures are trabeculectomy and drainage implant surgery.
A trabeculectomy involves making a flap-like opening in the sclera (the white part of the eye), under the eyelid. This is done to create a passageway for aqueous humor to drain.
The surgeon makes incisions through the conjunctiva and sclera to create the flap. Next, they create a tiny tunnel from inside the flap into the front of the eye. Finally, they lay the flap back into place and close it with loose stitches around the edges. This creates a little pocket, or "filter," to help regulate the amount of aqueous that flows out. A trabeculectomy usually takes less than an hour to complete.
In a glaucoma implant procedure, the surgeon creates a pocket between the conjunctiva and sclera. This is where the base of the implant will go.
While implants vary slightly, they all have a tiny, flexible tube attached to a thin plate. The plate rests inside the pocket, against the sclera. Then the tube is inserted through the sclera into the front of the eye. The implant allows aqueous fluid to flow through the tube and collect in the little plate until it’s naturally absorbed by the body. The procedure takes around an hour or less to complete.
It’s up to your eye doctor to determine whether you’re a good candidate for glaucoma surgery. If you do need surgery, they will help you determine which procedure is right for you.
LEARN MORE about glaucoma surgery
Risk factors
Certain characteristics put a person at higher risk of developing open-angle glaucoma. These characteristics include:
Having elevated IOP
Having a family history of POAG
Having Black or Latin American ancestry
Being 40+ years old
Having high myopia (severe nearsightedness)
Taking oral contraceptive pills
Having unmanaged anxiety and/or stress
Having Type 2 diabetes
Being obese
Consuming alcohol excessively
Having sleep apnea
Having a thinner-than-usual corneal thickness
Having blood pressure that’s too high or too low
Having migraine headache or vasospasm disorder
If you have one or more of these risk factors, it’s important to have comprehensive eye exams regularly.
Eye health experts recommend yearly eye exams for everyone over age 6, regardless of symptoms or risk factors. People with risk factors for eye disease may need them more often. The only way to ensure healthy vision and keep eye disease like glaucoma at bay is with regular eye exams.
When to see an eye doctor
See an eye doctor right away if you notice blurriness or shadow-like darkness in your peripheral vision. You should also see your eye doctor regularly if you have one or more risk factors for glaucoma.
If you have been diagnosed with glaucoma, following your treatment instructions is critical. This includes medication instructions and attending all of your scheduled check-ups. Your eye doctor needs to see you on a regular basis to make sure treatment is working properly.
Amber McManes also contributed to this article.
Types of glaucoma. Glaucoma Research Foundation. Accessed May 2023.
Don't let glaucoma steal your sight! Centers for Disease Control and Prevention. November 2020.
Glaucoma. Penn Medicine. August 2022.
Glaucoma and the importance of the eye’s drainage system. BrightFocus Foundation. July 2021.
What is primary open-angle glaucoma? Glaucoma Research Foundation. Accessed May 2023.
What is angle-closure glaucoma? Glaucoma Research Foundation. Accessed May 2023.
Primary open-angle glaucoma. Merck Manual Professional Version. April 2023.
Types of glaucoma. National Eye Institute. September 2021.
Primary open-angle glaucoma. American Academy of Ophthalmology. EyeWiki. March 2023.
What are the signs of open angle glaucoma? Keck Medicine of USC. January 2022.
Open-angle glaucoma: are there symptoms? BrightFocus Foundation. July 2021.
Glaucoma tests. MedlinePlus [Internet]. National Library of Medicine. December 2020.
Five common glaucoma tests. Glaucoma Research Foundation. August 2022.
Glaucoma tests. Cleveland Clinic. March 2022.
Glaucoma medicines. National Eye Institute. July 2021.
Treatments for open-angle glaucoma. BrightFocus Foundation. July 2021.
Acetazolamide. Drugs.com. September 2022.
Methazolamide. Drugs.com. October 2022.
Laser surgery. Glaucoma Research Foundation. March 2022.
Glaucoma surgery series: cyclophotocoagulation. BrightFocus Foundation. August 2021.
Role of minimally invasive glaucoma surgery in the management of chronic open-angle glaucoma. Scientific Reports. November 2021.
What are the benefits and risks of minimally invasive (Trabectome) surgery for treating glaucoma (a common eye condition)? Cochrane Database of Systematic Reviews. February 2021.
iStent. Wills Eye Hospital. Accessed May 2023.
Incisional surgery for glaucoma. Glaucoma Research Foundation. March 2022.
Glaucoma surgery. National Eye Institute. January 2022.
Trabeculectomy surgery for glaucoma. Wills Eye Hospital. Accessed May 2023.
What is a glaucoma drainage implant? American Academy of Ophthalmology. EyeSmart. April 2023.
Open angle glaucoma. StatPearls [Internet]. August 2022.
Page published on Thursday, July 23, 2020
Page updated on Tuesday, August 8, 2023
Medically reviewed on Saturday, June 3, 2023